Regulatory experts at Novagenx are adaptive, organized, and equipped with vast regulatory experties
 Regulatory Affairs and Operations
Regulatory Affairs and Operations
Industry-trained experts at Novagenx are skilled at managing, preparing, and submitting a diverse array of regulatory submissions to global health authorities, serving as an extension to the sponsor team. Throughout the product life-cycle, Novagenx experts provide high-quality and region-specific regulatory submission support using validated document management systems, local expertise, and eCTD publishing systems.
During the submission authoring and compilation process, Novagenx proactively engages proven processes to streamline activities, incorporate cross-departmental efficiencies, and build multiple health authority applications in parallel to execute regulatory submissions across the globe. Tenured colleagues can anticipate regulatory issues and resolve them in a timely, detailed manner.
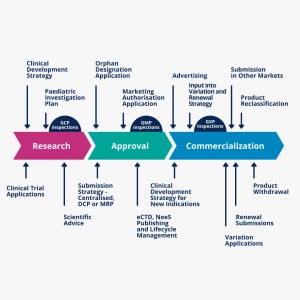
From preclinical to late-stage regulatory strategy and global alignment of submissions and dossiers, Novagenx provides:
- Early-stage regulatory operations, including applying for application numbers, filing an intent to submit applications, meetings and briefing packages support, and advisory meetings
- Clinical development regulatory operations and global submissions of study authorization applications, including initial study applications and amendments; scientific advice submissions; special designation submissions like fast-track designation, and special protocol assessment requests
- Marketing authorization and post-approval life-cycle management, including initial marketing authorization applications or amendments and the maintenance of approved marketing applications.