
Validation & Qualification
Computer System Validation
Data Integrity
Process Validation
Cleaning Validation
Equipment Qualification
Facilities Commissioning & Qualification
Aseptic Remediation and Operations Evaluations
Our Services

Computer System Validation
Data Integrity
Process Validation
Cleaning Validation
Equipment Qualification
Facilities Commissioning & Qualification
Aseptic Remediation and Operations Evaluations

Quality Systems Implementation
FDA Warning Letter/483 Remediation
FDA Readiness Inspections / Mock Audits
Quality System Audits / GAP Assessments
IT and Supplier Audits
CAPA, Deviation & Complaint Inv/Closeout
Regulatory Compliance Services
M&A Compliance / Due Diligence
Technology Transfer
Laboratories

Program & Project Management
Rapid Project Startup Services
Project Management Training
cGMP Training
Customized Training Programs

dossiers, Novagenx provides:
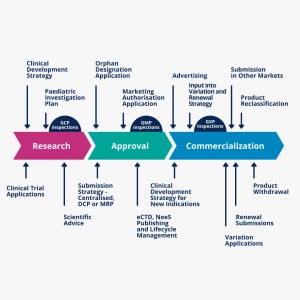
1-Early-stage regulatory operations, including applying for application numbers, filing an intent to submit applications, meetings and briefing packages support, and advisory meetings
2-Clinical development regulatory operations and global submissions of study authorization applications, including initial study applications and amendments; scientific advice submissions; special designation submissions like fast-track designation, and special protocol assessment requests
3-Marketing authorization and post-approval life-cycle management, including initial marketing authorization applications or amendments and the maintenance of approved marketing applications.
-An experienced team to help manage studies across a variety of industry standard EDC platforms;
-Expertise to recommend and deploy cutting-edge “e” or electronic technologies including ePRO, eCOA and eConsent with minimal expense and no disruptions;
-A wide range of therapeutic area experience;
-proven processes to begin clinical studies faster, with accurate, clean results more quickly;
near real-time access to clean clinical data; and
-A single point of contact, allowing sponsors to spend less time managing vendors and more time on trial analysis and insights

HELPING OUR PARTNERS TO SOURCE, ENGAGE AND MOBILIZE HIGH-CALIBER PROFESSIONALS FOR SPECIALIST VACANCIES ACROSS THE GLOBE